Capsids and Pore-forming Toxins
Engineering of protein capsids
Compartmentalization is a fundamental concept in every organism and considerable efforts are being undertaken to engineer artificial compartments for diverse biomedical and biotechnological applications such as drug delivery, catalysis and bio-imaging.
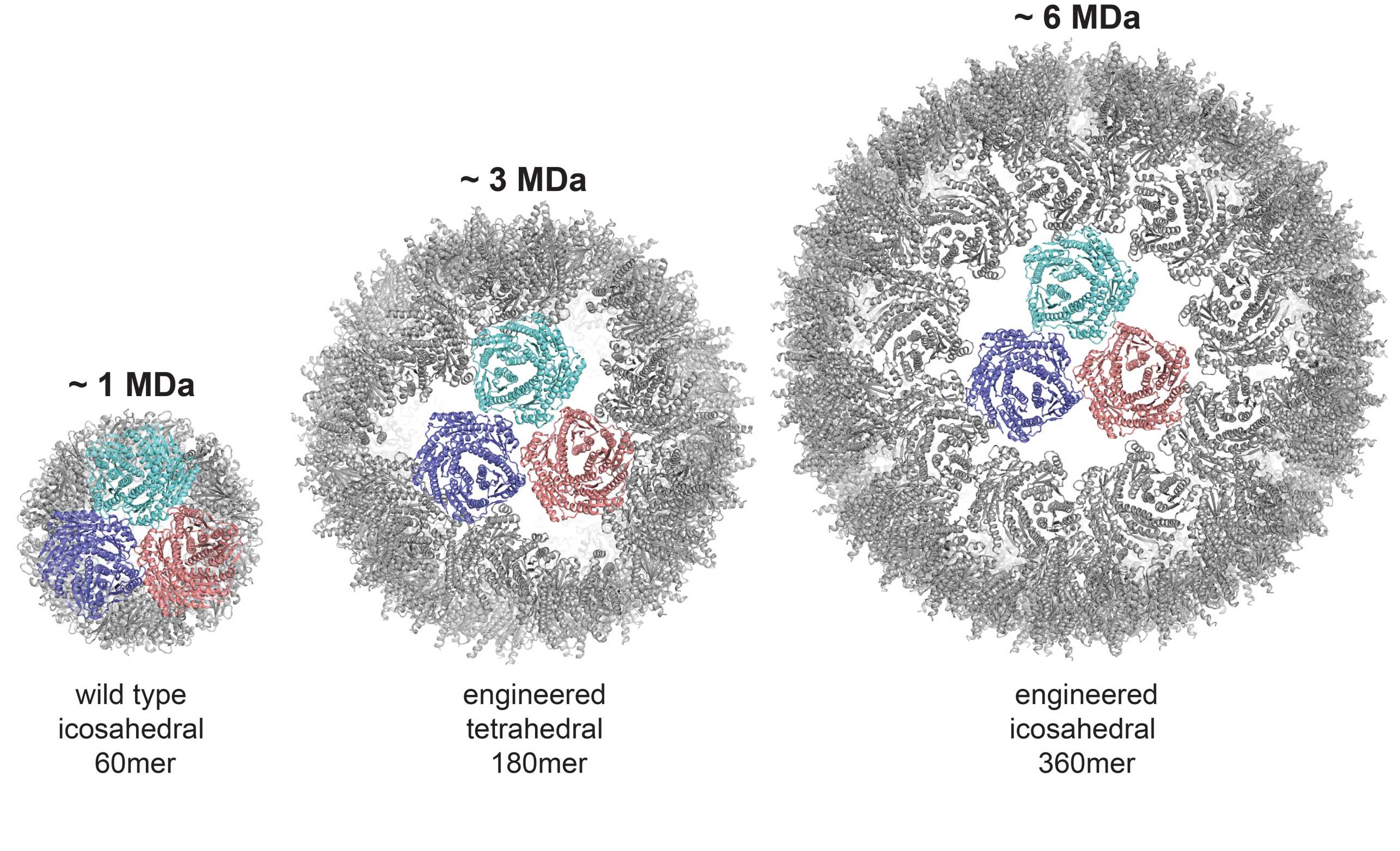
In collaboration with the Hilvert Group, we determined the cryo-EM structures of two versatile encapsulation systems (external page Sasaki et al. 2017), which were engineered based on the naturally occurring lumazine synthase, a protein that assembles into a ~ 1 MDa dodecahedron composed of 60 monomers (corresponding to 12 pentamers). Introducing additional negative charges into the monomers lead to slight changes in the overall shape of the pentamers, which resulted in self-assembling ~ 3 MDa and ~ 6 MDa capsids with unprecedented tetrahedral and icosahedral symmetries that consisted of either 80 monomers (36 pentamers) or 360 monomers (72 pentamers), respectively.
Enzyme Encapsulation
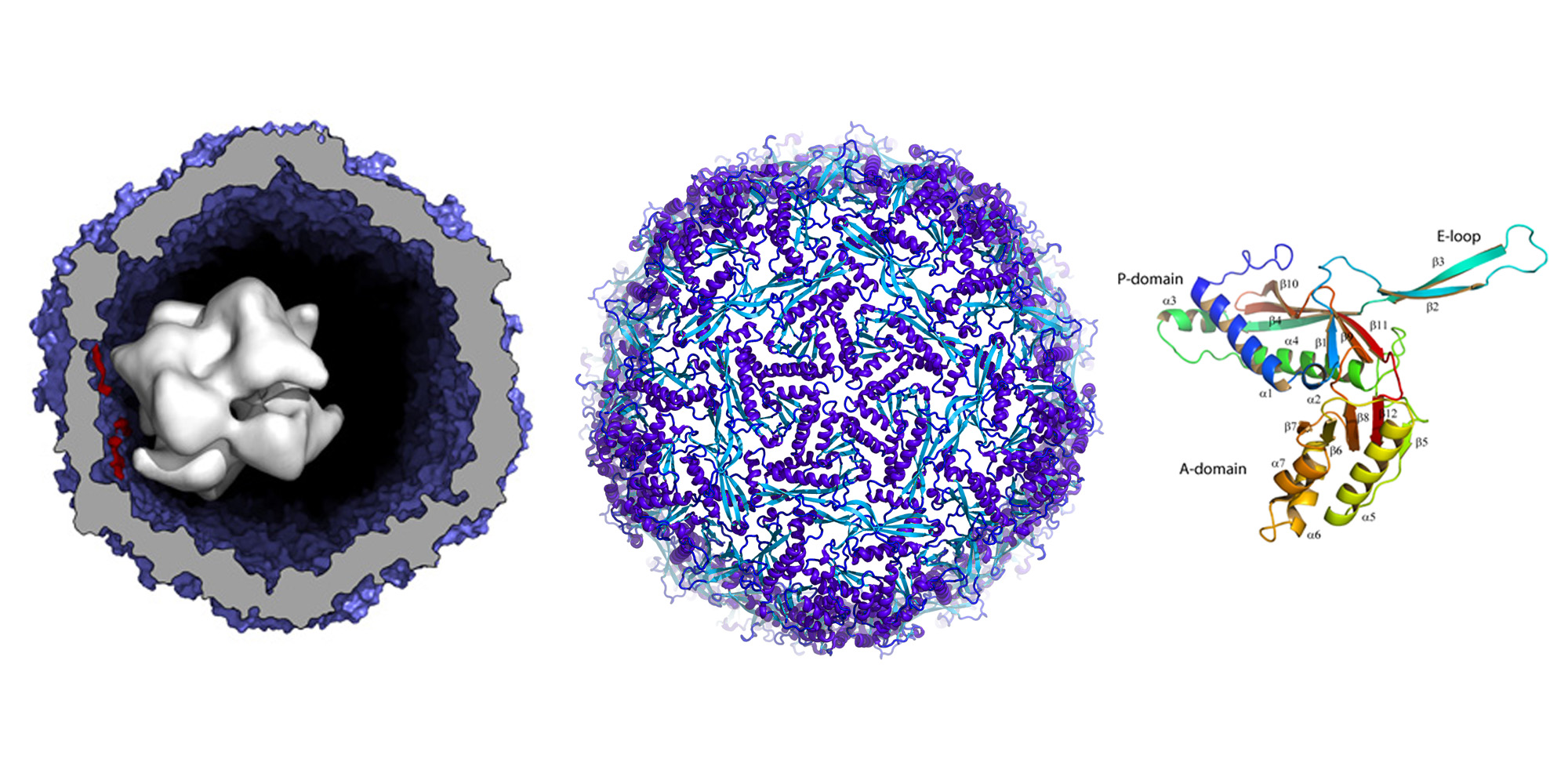
Compartmentalization is an important organizational feature of life. It occurs at varying levels of complexity ranging from eukaryotic organelles and bacterial microcompartments to the molecular reaction chambers formed by enzyme assemblies. Using X-ray crystallographic, biochemical and EM experiments, we demonstrated that a widespread family of conserved bacterial proteins, the linocin-like proteins, form large assemblies that function as a minimal compartment to package enzymes (external page Sutter et al. 2008). We refer to this shell-forming protein as 'encapsulin'. The crystal structure of such a particle from Thermotoga maritima determined at 3.1 Å resolution reveals that 60 copies of the monomer assemble into a thin, icosahedral shell with a diameter of 240 Å. The interior of this nanocompartment is lined with conserved binding sites for short polypeptide tags present as C-terminal extensions of enzymes involved in oxidative-stress response.
Pore-forming toxins
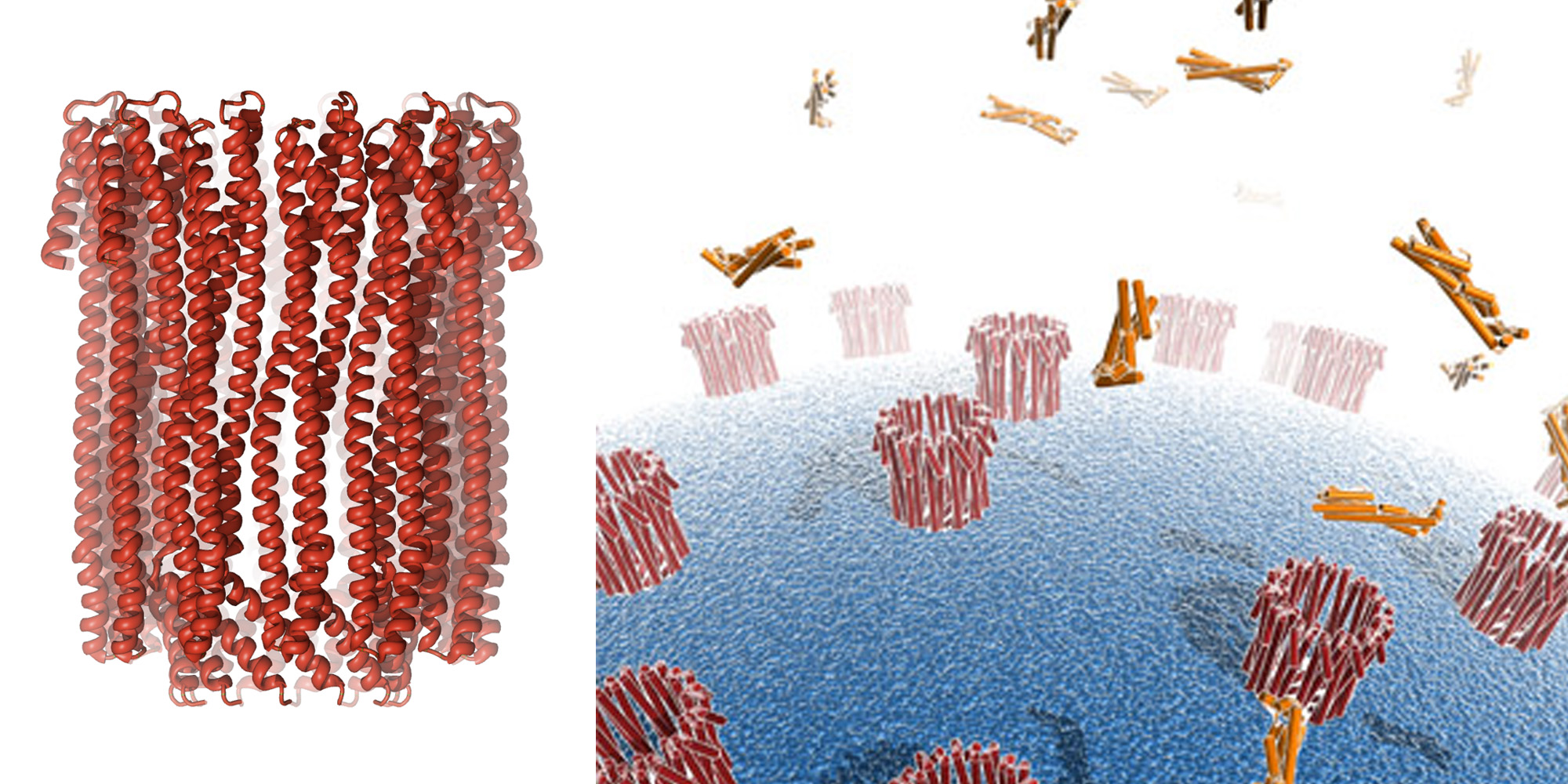
Pore-forming toxins are potent virulence factors that convert from a soluble form to a membrane-integrated pore and include some of the most dangerous toxins, such as diphtheria and anthrax toxin. They exhibit their toxic effect either by destruction of the membrane permeability barrier or by delivery of toxic components through the pores. Eukaryotic examples of pore-forming toxins are the immune system proteins perforin and the membrane-attack complex. Cytolysin A (ClyA, also known as HlyE) is a cytolytic alpha-helical toxin responsible for the haemolytic phenotype of several Escherichia coli and Salmonella enterica strains.
Our 3.3 Å crystal structure of the 400 kDa dodecameric transmembrane pore formed by ClyA reveals that the tertiary structure of ClyA protomers in the pore is substantially different from that in the soluble monomer (external page Mueller et al. 2009, external page Mueller and Ban 2010). The conversion involves more than half of all residues and results in large rearrangements of parts of the monomer, reorganization of the hydrophobic core, and transitions of beta-sheets and loop regions to alpha-helices. The large extent of interdependent conformational changes indicates a sequential mechanism for membrane insertion and pore formation.