Fatty Acid Synthases
Fatty acids are used in the cell as energy storage compounds and as messenger molecules. Their biosynthesis is an essential process for most organisms and follows a conserved pathway for stepwise precursor elongation by two-carbon carboxylic acid building blocks in several reaction cycles, involving six different enzymes and a carrier protein for substrate shuttling.
While in most bacteria and plants, each reaction step is catalyzed by an individual enzyme that is dissociated from the others, fatty acid synthesis in other higher organisms is catalyzed by large multifunctional proteins, where many individual enzymes are fused together to form a “molecular assembly line” (external page Maier et al. 2010). We studied the architecture of two representative classes of multifunctional fatty acid synthases (FAS): The fungal and mammalian systems reveal two different architectural solutions that allow these giant multi-enzymes to hand over the products of one enzymatic active site directly to the next (“substrate channeling”).
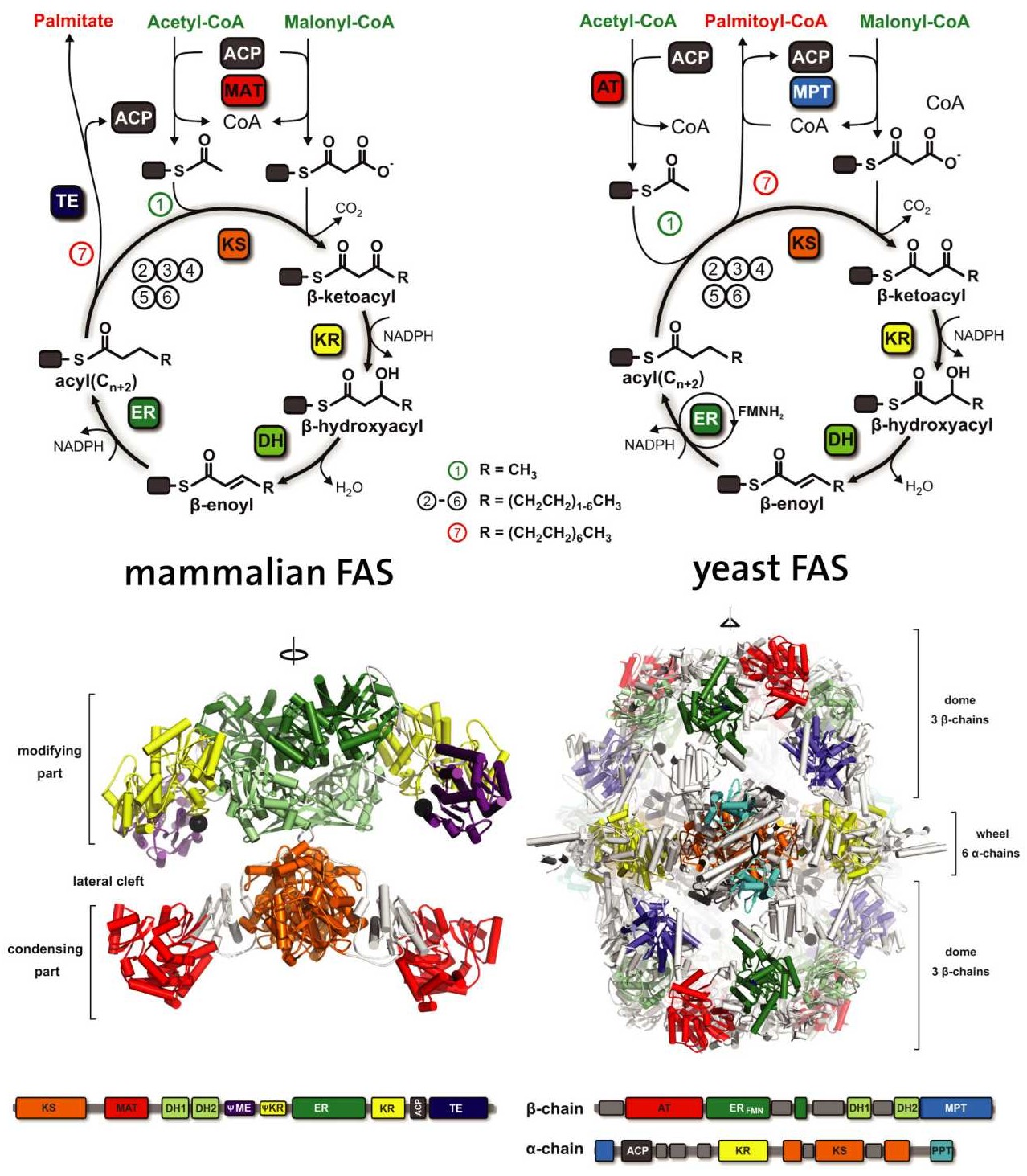
We solved the crystal structure of fungal FAS, a huge 2.6 MDa heterododecameric assembly (external page Jenni et al. 2006, external page Jenni et al. 2007, external page Leibundgut et al. 2007). The structure suggest how the growing fatty acid reaction intermediates are shuttled by the flexibly attached acyl carrier domain (ACP) between the active sites inside a barrel shaped reaction chamber. During the reaction cycles, the acyl chain bound to ACP adopts an extended conformation, as shown in an NMR study performed in cooperation with the Wider lab (external page Perez et al. 2015), while in bacteria, the acyl chain is buried within the ACP when shuttled over larger distances from one enzyme to the next. A multimeric FAS related to the fungal system exists also in some bacterial genera, and we reconstructed the structure of mycobacterial FAS using cryo-electron microscopy (external page Boehringer et al. 2013).
A different architecture for FAS was revealed by the structure of the mammalian FAS, a dimer of 540 KDa, in which the catalytic domains are arranged in two semicircular reaction clefts (external page Maier et al. 2006, external page Maier et al. 2008). The essential FAS has a central role in the primary metabolism of mammalian cells and is considered a promising drug target for treatment of cancer and other pathologies. The structure of the mammalian FAS also provided insights into the architecture of evolutionary related bacterial polyketide synthases, enzymes that produce a vast variety of structurally diverse natural compounds such as antibiotics.