Ribosome Assembly
Structures of the mature ribosomal subunits in different functional states have been available for some years now, but our knowledge about their assembly at atomic resolution is only emerging. As the composition of the mature ribosomes of eukaryotes, prokaryotes and organelles diverged through evolution, so did their assembly pathways, adapting to the presence of rRNA expansion segments, new or modified ribosomal proteins, and the need to travel through organelle membranes.
Assembly of mitochondrial ribosomes
Mitochondrial ribosomes, specialized for synthesizing membrane proteins crucial for oxidative phosphorylation, have undergone significant divergence from their bacterial ancestors. Due to their distinct architectural features and the need to synchronize mitochondrial ribosomal RNA synthesis with the import of mitoribosomal proteins, their assembly is expected to engage mitochondrial-specific pathways and involve both conserved and mitochondrial-specific assembly factors.
Assembly of human mitochondrial ribosomes
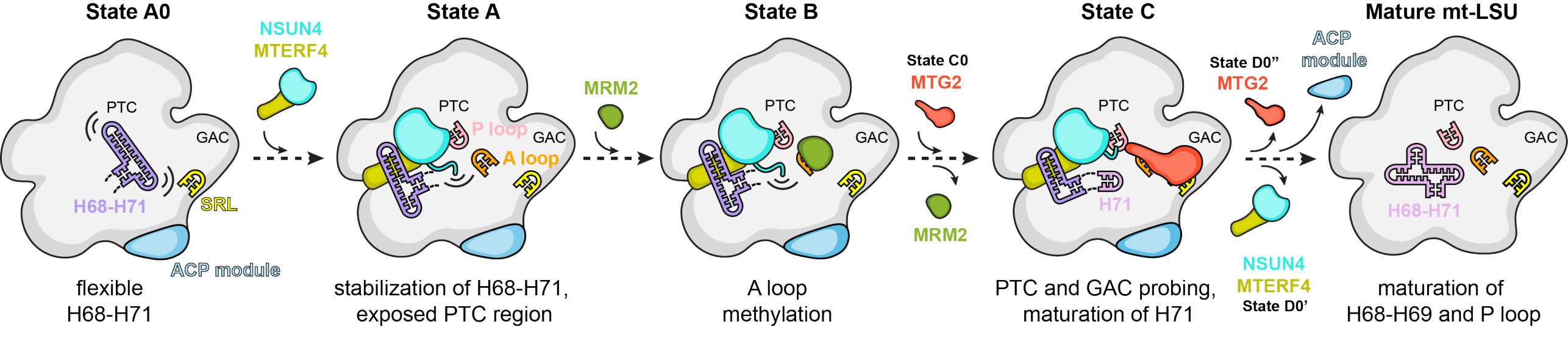
In this study (external page Lenarcic et al. 2021), we unveiled eight distinct late-stage assembly stages of the human large mitoribosomal subunit. In collaboration with the group of external page Aleksandra Filipovska lab, we identified the NSUN4-MTERF4 dimer's pivotal role in this process. The dimer stabilizes the 16S rRNA in a configuration that exposes functionally critical regions for modification by the MRM2 methyltransferase. Additionally, it facilitates peptidyl transferase center quality control together with the conserved mitochondrial GTPase MTG2 (Obg domain), which concomitantly interacts with the sarcin-ricin loop with its G domain. The consecutive engagement of these factors triggers the maturation of the peptidyl transferase active site of the mitoribosome, along with the proper folding of adjacent rRNA regions essential for interactions with tRNAs and the small ribosomal subunit.
Assembly of trypanosomal mitochondrial ribosomes
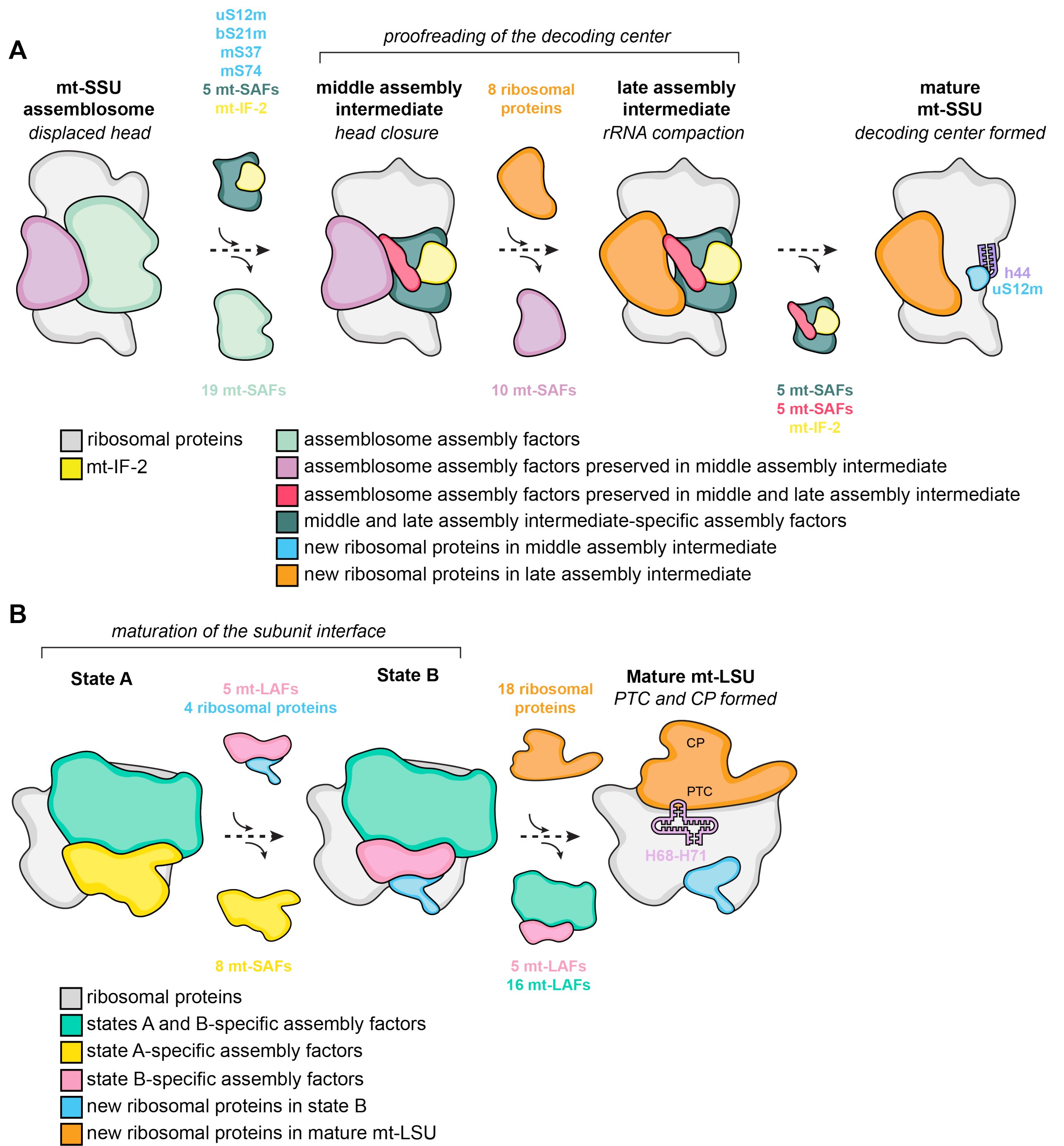
A striking example of evolutionary changes is found in the mitoribosome from Trypanosoma brucei, a parasitic kinetoplastid responsible for African trypanosomiasis or sleeping sickness in humans, where a drastic reduction in rRNA size triggers an architectural shift, favoring mitoribosomal proteins over their bacterial counterparts. Teamed up with the external page Schneider lab we aimed to better understand the intricacies of its assembly process. We investigated both native and affinity-tagged assembly intermediates of the mitoribosomal small and large subunits using cryo-electron microscopy (external page Saurer et al. 2019, external page Jaskolowski et al. 2020, external page Lenarcic et al. 2022). In total, we identified 28 assembly factors for the large subunit and 39 for the small subunit maturation, including the involvement of mitochondrial initiation factor 2. These factors interact with the partially folded rRNA, selectively recognizing critical ribosomal regions, such as the peptidyltransferase center and the decoding center. Comparison of the architectural and compositional characteristics of these assembly stages unveiled a modular assembly process, guiding the rRNA towards its mature configuration.
Eukaryotic Ribosome Biogenesis
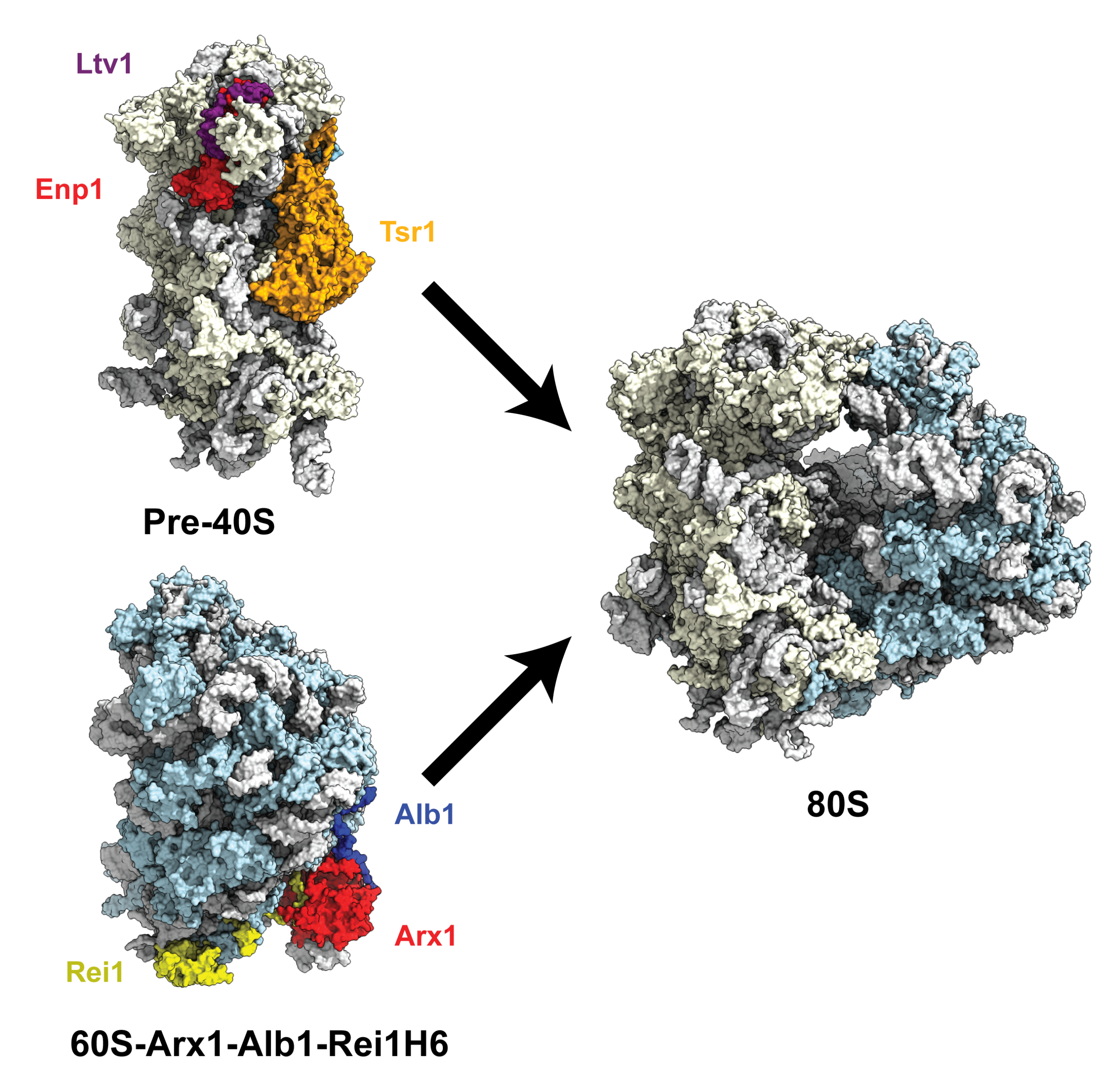
In eukaryotes, ribosome assembly is a complex process involving more than 200 assembly factors and spreads from the nucleolus to the cytoplasm. The process starts with the synthesis by the RNA Pol-I of the 35S pre-rRNA, which is co-transcriptionally bound and folded by ribosomal proteins and assembly factors. Cleavage of this emerging rRNA releases the earliest small ribosomal subunit precursor, the 90S. The remaining rRNA will continue to be synthetized and become the large ribosomal subunit precursor.
To understand the pathway between those precursors and the mature ribosomes, our group, in collaboration with the group of external page Vikram Panse at the University of Zurich, visualized intermediates, revealing the interactions of assembly factors with the immature ribosomes. Those structures displayed how the assembly factors probe the integrity of important regions of the ribosome and lock the ribosomal proteins and the rRNA in an immature conformation, preventing premature progression through the maturation process. Additionally, the assembly factors were also shown to hamper the immature ribosomal subunits to take part in a possibly error-prone translation by shielding the functional regions of the ribosomal subunits.